Introduction
In the realm of assisted reproductive technology (ART), in vitro fertilization (IVF) has become a well-established procedure, offering pathways to parenthood for many. Emerging from this field is mitochondrial replacement therapy (MRT), a groundbreaking technique also referred to as mitochondrial donation or, less accurately, three-parent IVF.
This innovative approach uniquely involves the genetic material of three individuals to address a critical health concern: the transmission of inherited mitochondrial diseases. These conditions, stemming from defects in the mitochondria—the energy powerhouses of our cells—can lead to severe and often fatal health issues.
The purpose of this article is to provide a clear and comprehensive examination of mitochondrial replacement therapy (MRT), delving into the underlying science, exploring its potential benefits in preventing devastating mitochondrial dysfunction, and carefully considering the complex ethical considerations that surround its application.
As our understanding of genetics advances, mitochondrial replacement therapy (MRT) has become increasingly relevant, sparking significant discussion about its role in reproductive medicine and the societal implications of manipulating the human germline to prevent disease.
Decoding the Genetics: Why Mitochondrial Replacement Therapy (MRT)?
To understand the rationale behind Mitochondrial Replacement Therapy (MRT), it’s crucial to appreciate the unique role of mitochondria and mtDNA inheritance. Unlike the majority of our genetic information housed in the nucleus of our cells, mitochondrial DNA resides within the mitochondria themselves and is passed down exclusively from the mother.
Consequently, mutations in this mtDNA can lead to a range of debilitating conditions collectively known as mitochondrial diseases. These diseases can manifest in diverse and severe ways, affecting vital organs such as the heart, liver, and brain, often resulting in significant morbidity and reduced lifespan.
The prevalence of serious inherited mitochondrial diseases in newborns underscores the urgency for effective preventative strategies. Mitochondrial Replacement Therapy (MRT) directly targets this mode of inheritance.
The Techniques of Mitochondrial Replacement Therapy (MRT): MST vs. PNT
Currently, two primary techniques fall under the umbrella of Mitochondrial Replacement Therapy (MRT): Maternal Spindle Transfer (MST) and Pronuclear Transfer (PNT).
- Maternal Spindle Transfer (MST): This procedure, a key method in Mitochondrial Replacement Therapy (MRT), involves meticulously extracting the nuclear DNA—which holds the vast majority of the mother’s genetic blueprint—from her egg that contains faulty mitochondria. Simultaneously, the nuclear DNA is removed from a healthy donor egg, which possesses healthy donor mitochondria.
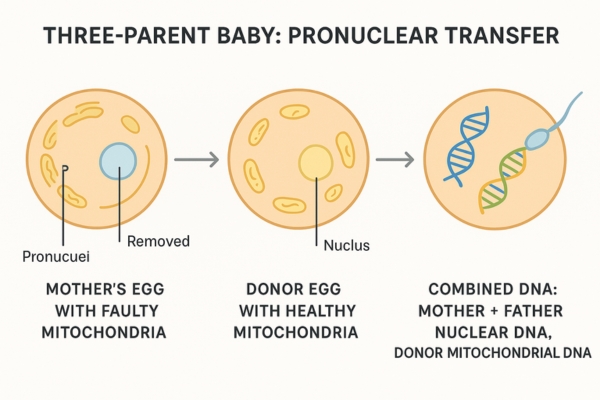
The mother’s nuclear DNA is then carefully inserted into the donor egg, effectively replacing the unhealthy mitochondria while retaining the mother’s core genetic identity. This reconstructed egg, now containing the mother’s nuclear DNA and healthy donor mitochondria, is then fertilized with the father’s sperm, leading to embryo development free from the mother’s mitochondrial defect. - Pronuclear Transfer (PNT): This alternative Mitochondrial Replacement Therapy (MRT) technique begins with the fertilization of both the mother’s egg (with faulty mitochondria) and a healthy donor egg (with healthy mitochondria) with the father’s sperm. Before the fertilized eggs begin to divide, the pronuclei—which contain the combined nuclear DNA from the sperm and egg—are removed from both.
The pronuclei from the parents’ fertilized egg are then transferred into the donor’s fertilized egg, whose own pronuclei have been removed. The resulting embryo, a product of Mitochondrial Replacement Therapy (MRT), carries the parents’ nuclear DNA and the donor’s healthy mitochondrial DNA.
The Result of Mitochondrial Replacement Therapy (MRT): Genetic Contribution
It is essential to clarify the genetic contributions in Mitochondrial Replacement Therapy (MRT). The resulting child inherits approximately 99.9% of their genetic material, specifically the nuclear DNA, from their intended mother and father. The mitochondrial donor contributes a minuscule amount, around 0.1%, in the form of healthy mitochondrial DNA.
This distinction is central to the ongoing debate about whether individuals conceived through Mitochondrial Replacement Therapy (MRT) effectively have three genetic parents. While the donor provides essential healthy components, the core of the child’s genetic makeup originates from the intended parents.
Preventing the Transmission of Inherited Mitochondrial Diseases with MRT
The most significant benefit of Mitochondrial Replacement Therapy (MRT) lies in its potential to offer a direct pathway for families with a history of mitochondrial disease to conceive healthy children. For couples who know they carry the genetic burden of these often-devastating conditions, MRT presents a chance to break the cycle of inheritance.
Consider, for instance, Leigh syndrome, a severe neurological disorder, or MELAS syndrome, which affects the brain and muscles—both are examples of specific mitochondrial diseases that MRT could help prevent. By ensuring the offspring inherit healthy donor mitochondria, this technology holds the promise of eliminating the profound suffering, developmental delays, and premature death frequently associated with mitochondrial dysfunction.
While preimplantation genetic diagnosis (PGD) is an option for some genetic conditions, it may not always be a viable or effective alternative for severe mtDNA mutations. PGD involves testing embryos created through standard in vitro fertilization (IVF) for genetic defects before implantation. However, with mitochondrial DNA, a phenomenon called heteroplasmy complicates matters.
Heteroplasmy refers to the presence of both mutated and healthy mtDNA within the same cell. The proportion of mutated mtDNA can vary significantly between embryos, making it challenging to accurately predict which embryos will be severely affected. In cases of high heteroplasmy, even embryos deemed “low risk” by PGD might still develop the disease. In contrast, MRT aims to replace virtually all of the mother’s faulty mitochondria with healthy donor mitochondria, offering a more definitive approach to preventing disease transmission.
Offering Hope and Maintaining Genetic Link with MRT
The development of Mitochondrial Replacement Therapy (MRT) represents a significant advancement beyond earlier attempts to address mitochondrial issues in reproduction. For example, ooplasmic transfer (cytoplasmic transfer), an earlier technique, involved injecting cytoplasm from a donor egg into the mother’s egg to improve egg quality. However, this approach raised safety concerns and lacked the targeted genetic precision of Mitochondrial Replacement Therapy (MRT).
Crucially, Mitochondrial Replacement Therapy (MRT) allows the intended parents to have a child who is genetically related to them through their nuclear DNA. This is a significant distinction from traditional egg donation, where the child shares no genetic link with the intended mother.
For many couples facing the prospect of transmitting a severe mitochondrial disease, the ability to have a child who carries their nuclear genes is of profound emotional and psychological importance. This genetic link to parents, facilitated by Mitochondrial Replacement Therapy (MRT), can be a crucial factor in their decision-making process, offering a sense of biological continuity that other reproductive options may not provide.
The “Three-Parent Baby” Debate and Mitochondrial Replacement Therapy (MRT)
One of the most prominent ethical implications surrounding Mitochondrial Replacement Therapy (MRT) is the question of whether a child born through this procedure has three genetic parents. While the child inherits the vast majority of their genetic material from the intended mother and father, the contribution of mitochondrial DNA from the donor raises complex questions about parenthood and genetic identity.
Proponents of Mitochondrial Replacement Therapy (MRT) emphasize the minimal genetic material transfer from the mitochondrial donor, arguing that the donor’s contribution is limited to a small fraction of the overall genome and primarily concerns cellular energy production, not the traits that define an individual’s personality or appearance.
Conversely, those with ethical reservations highlight the biological significance of mtDNA in cellular function and development. They argue that even a small amount of genetic material from a third party complicates traditional notions of family and lineage, leading to the moniker “three-parent IVF.”
The ongoing debate reflects differing perspectives on the relative importance of nuclear versus mitochondrial DNA in defining genetic parentage and the potential psychological impact on the child of knowing they have a third genetic contributor. The legal and social ramifications of acknowledging a mitochondrial donor as a “parent” are also subjects of considerable discussion in the context of Mitochondrial Replacement Therapy (MRT).
Safety and Long-Term Effects of Mitochondrial Replacement Therapy (MRT) on the Child
Given the novelty of Mitochondrial Replacement Therapy (MRT) techniques, concerns about the long-term safety and development of children born through this method are paramount. While initial outcomes appear promising, the potential for unforeseen health issues or complex genetic interactions resulting from the combination of the parents’ nuclear DNA and the donor’s mitochondrial DNA remains a subject of ongoing investigation.
It is crucial that rigorous research and long-term monitoring of children born through Mitochondrial Replacement Therapy (MRT) are conducted to fully understand any potential health consequences. This commitment to safety is a crucial aspect of responsible Mitochondrial Replacement Therapy (MRT) implementation.
The history of ooplasmic transfer, which was associated with some adverse outcomes, serves as a cautionary tale, emphasizing the need for stringent safety protocols and thorough preclinical testing before the widespread application of Mitochondrial Replacement Therapy (MRT). Ensuring the well-being of children born through this technology is a central ethical consideration.
Germline Modification and the “Slippery Slope” Argument Regarding MRT
Mitochondrial Replacement Therapy (MRT) inherently involves germline modification, meaning that the genetic alteration (the replacement of mitochondria) can be passed on to future generations. This aspect raises significant ethical concerns surrounding the intentional modification of the human genome. Critics worry about the potential for unintended consequences for future generations stemming from these alterations, even if the initial aim is to prevent disease.
The “slippery slope” argument is frequently invoked in discussions about Mitochondrial Replacement Therapy (MRT). This concern posits that legalizing MRT, even for the noble purpose of preventing serious mitochondrial diseases, could inadvertently pave the way for more extensive and controversial genetic modifications in the future, potentially leading to non-therapeutic enhancements or “designer babies.” Careful consideration of the boundaries and regulations surrounding germline editing is therefore essential in the ethical evaluation of MRT.
Concerns for the Egg Donor in Mitochondrial Replacement Therapy (MRT)
The process of egg donation, which is integral to Mitochondrial Replacement Therapy (MRT), carries both physical and emotional risks for the third party involved. These risks include those associated with ovarian stimulation and egg retrieval.
Furthermore, issues of compensation, anonymity, and the potential for the exploitation of donors in the context of Mitochondrial Replacement Therapy (MRT) need careful consideration. Robust ethical guidelines and regulations are necessary to ensure the fair and ethical treatment of egg donors involved in Mitochondrial Replacement Therapy (MRT), protecting their rights and well-being.
Societal Implications and Access to Mitochondrial Replacement Therapy (MRT)
The advent of Mitochondrial Replacement Therapy (MRT) has broader social implications regarding germline editing. It prompts discussions about evolving definitions of family structures and societal perceptions of genetic inheritance. Moreover, the potential cost of this advanced technology raises concerns about equitable access.
If Mitochondrial Replacement Therapy (MRT) becomes widely available, it is crucial to consider how to ensure that it is accessible to all eligible families, regardless of their socioeconomic status. The legal aspects of three-parent IVF and the diverse regulatory landscape surrounding Mitochondrial Replacement Therapy (MRT) across different countries highlight the lack of global consensus on this complex issue.
While the UK has cautiously legalized Mitochondrial Replacement Therapy (MRT) for specific medical reasons, many other nations maintain restrictions or outright prohibitions, reflecting the ongoing ethical and societal debates.
Conclusion
In conclusion, Mitochondrial Replacement Therapy (MRT) represents a significant scientific advancement with the potential to revolutionize the prevention of devastating inherited mitochondrial diseases. By offering a pathway to healthy offspring for families at risk, MRT provides tangible hope in the face of often-untreatable conditions caused by mitochondrial dysfunction.
However, the application of this technology is intertwined with complex ethical considerations. The debate surrounding the concept of “three-parent IVF,” concerns about long-term safety, and the potential for unintended effects on the child, as well as the broader societal implications of germline modification, necessitate careful and ongoing scrutiny.
As we move forward, it is imperative to balance the remarkable potential of MRT to alleviate suffering with a robust commitment to reproductive ethics and responsible scientific innovation. The future of MRT will depend on our ability to navigate these complexities thoughtfully, ensuring that its application is both safe and ethically sound.
What exactly is Mitochondrial Replacement Therapy (MRT)?
Mitochondrial Replacement Therapy (MRT) is a specialized assisted reproductive technology (ART) designed to prevent the transmission of mitochondrial diseases from mother to child. It involves replacing the mother’s eggs with faulty mitochondria with healthy mitochondria from a mitochondrial donor egg, while retaining the mother’s nuclear DNA.
How does Mitochondrial Replacement Therapy (MRT) differ from traditional IVF?
Traditional IVF involves fertilization of the mother’s egg with the father’s sperm. Mitochondrial Replacement Therapy (MRT) is unique because it incorporates genetic material from a third party – the mitochondrial donor – to ensure the resulting embryo has healthy mitochondria and prevent mitochondrial dysfunction.
Is a child born through Mitochondrial Replacement Therapy (MRT) genetically related to three people?
Genetically, the child inherits the vast majority of their DNA (nuclear DNA) from their mother and father. They inherit a very small amount of DNA (mitochondrial DNA) from the donor. While there are three genetic contributors, the nuclear DNA, which determines most traits, comes from the parents. The ethical implications of this “three-parent baby” concept are still debated.
Can Mitochondrial Replacement Therapy (MRT) cure existing mitochondrial diseases in individuals?
No, Mitochondrial Replacement Therapy (MRT) is a preventative measure aimed at ensuring future generations do not inherit mitochondrial diseases. It does not treat individuals who already have these conditions or address existing mitochondrial dysfunction.
What are the potential risks associated with Mitochondrial Replacement Therapy (MRT)?
As a relatively new technology, the long-term safety and potential health effects on children born through Mitochondrial Replacement Therapy (MRT) are still being studied. There are also risks associated with the egg donation process for the mitochondrial donor. Ongoing research is crucial for assessing these risks.
Is Mitochondrial Replacement Therapy (MRT) legal everywhere?
No, the legal aspects of three-parent IVF vary significantly worldwide. Mitochondrial Replacement Therapy (MRT) is legal under specific circumstances in the UK, but faces restrictions or is prohibited in many other countries due to ethical concerns surrounding germline modification and the novelty of the procedure.